For healthcare institutions
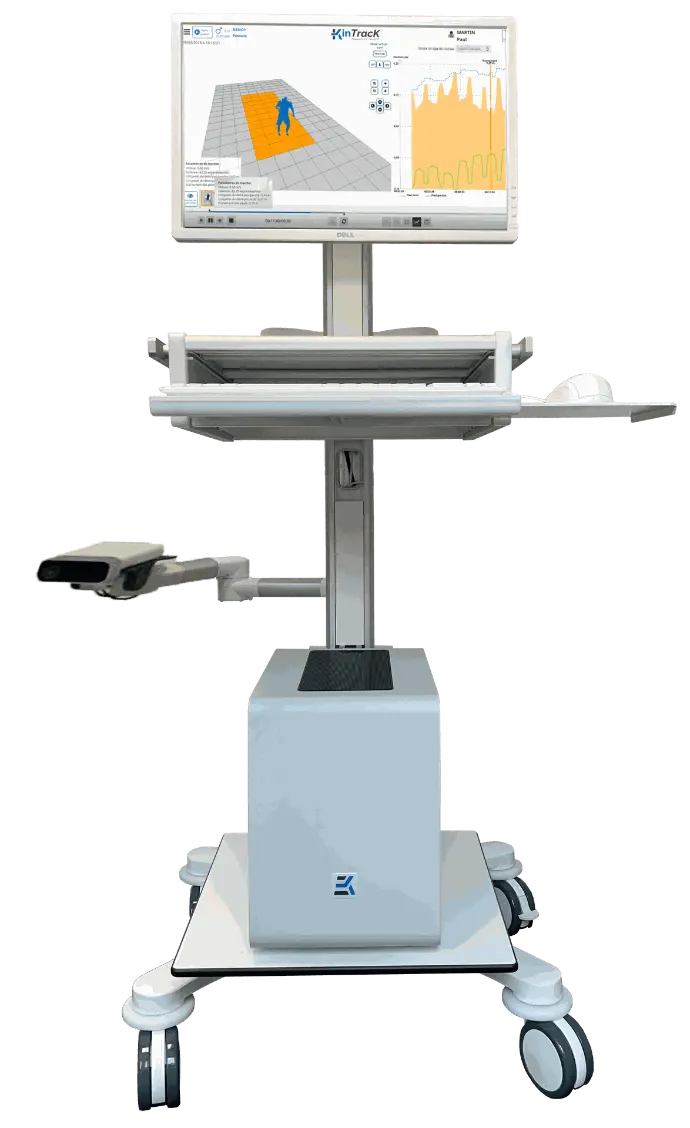
In the form of a medical cart...
... specifically designed to ease the use in physical rehabilitation institutions, this hardware is:
- Easily moveable, flexible, plug'n'play,
- Certified as a Medical Device, it respects the constraints in terms of stability, robustness, manufacturing materials, ...
- Equipped with a computer, a camera, a screen/keyboard/mouse, an 5-meter resistant electric cable (construction sites standards), and KinTracK software (see functionalities on the dedicated page).
In some countries, eligible for financing, contact us for more information (PTS of level 1 in France)
For what usages/patients?
Some examples...

Neurology
Post-stroke, Parkinson, multiple sclerosis, cerebral palsy
Initial, intermediary, final check-up
Botulinum toxin injections
Orthopedy
Traumatology, spinal cord injury, amputees
Before/after, with or without technical aids
Evaluation and adjustment of prosthesis/orthosis
Geriatry
Sarcopenia, falling risks
Regular assessment, preventive actions
(prescriptions for canes, rollator, home adaptations)
Performance
More generally, for anyone looking for recovery or improvement of physical performances (i.e., post injury athletes)
Distributed by the reference in France
Since 2020, EKINNOX delegates the distribution of KinTracK towards french rehabilitation institutions to the leader for high-end rehabilitation devices, Médimex.
For any request for a demo or sales proposition in France, do not hesitate to contact them directly.